
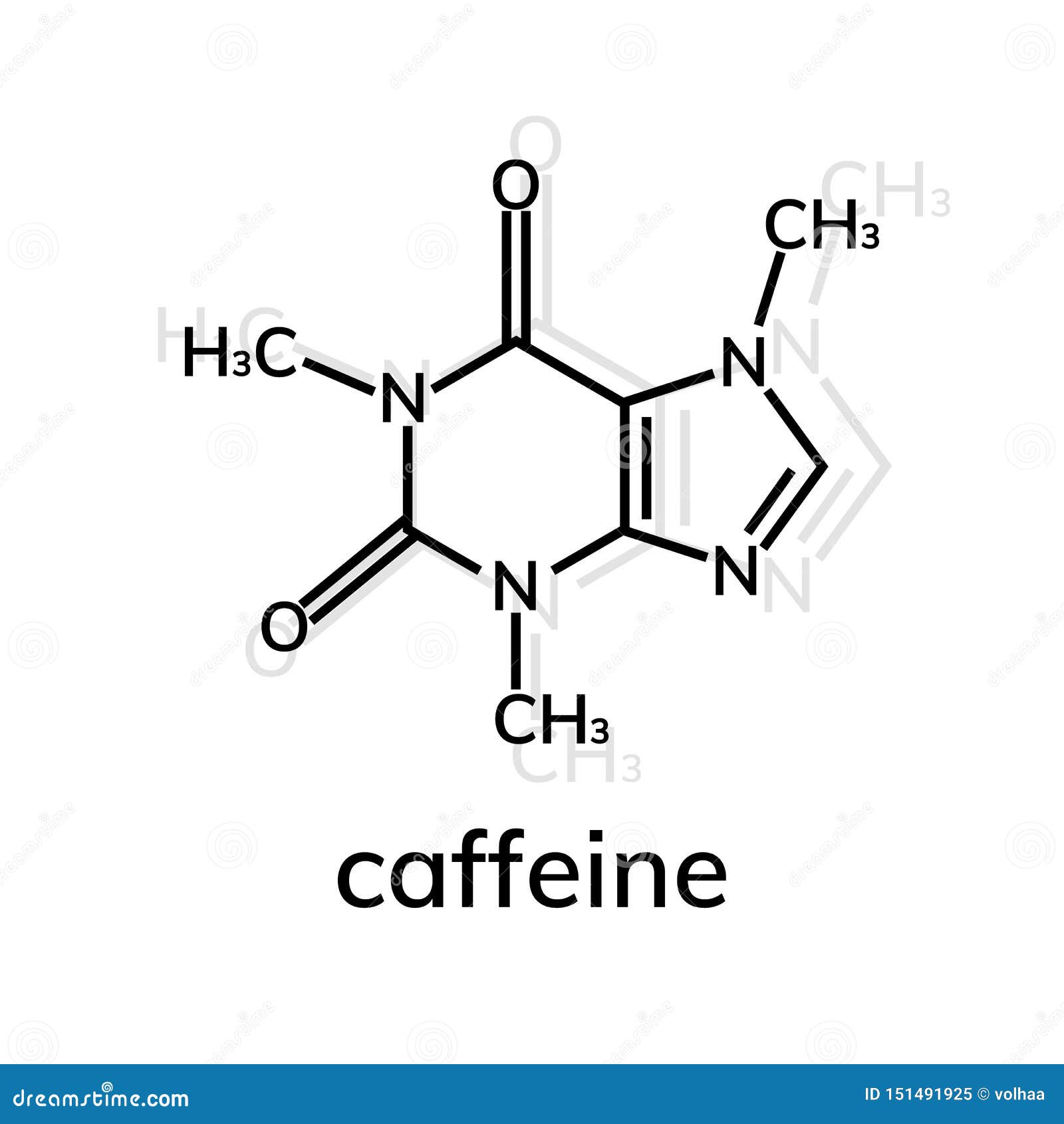
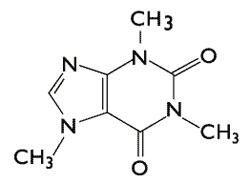
But the true difference in the compounds lies at the molecular level. Coffee (right) is made from Coffea Arabica, or coffee beans, and seeds and contains caffeine (Photo credit: JIhopgood/Flickr). įigure 2: Chocolate (left) is made from Theobroma cacao, or cacao plant seeds and contains theobromine (PC: Nic Charalambous). Theobromine produces mild positive effects in pleasure, but does not affect attention or alertness in moderate doses compared to caffeine. In behavioral studies, caffeine intake improves self-reported alertness and mood over a period of 24 hours. While these two compounds have similar effects, the key difference is that caffeine has an effect on the central nervous system and theobromine most significantly affects smooth muscle. Much like caffeine, theobromine is a diuretic however it mainly acts as a smooth muscle relaxant and cardiac stimulant. Chocolate, or theobromine, is found in products of Theobroma cacao, or cocoa plant seeds (Figure 2). It is predominantly a central nervous stimulant, though it also stimulates cardiac and skeletal muscles and relaxes smooth muscles. So how do these two molecules act on different parts of the body, making coffee the substance of choice over chocolate bars when midterm season hits? Caffeine is mostly derived from Coffea Arabica, or coffee beans, and seeds. In the case of caffeine, it turns out that the extra methyl group on the molecule is what makes coffee active on our central nervous systems and an “energy stimulator,” while chocolate functions as a sweet treat and smooth muscle stimulator.įigure 1: During the metabolism of caffeine in the body, the methyl group (highlighted by the yellow box) is removed from caffeine and it is converted to theobromine (Modified from Wolf LK, 2013).
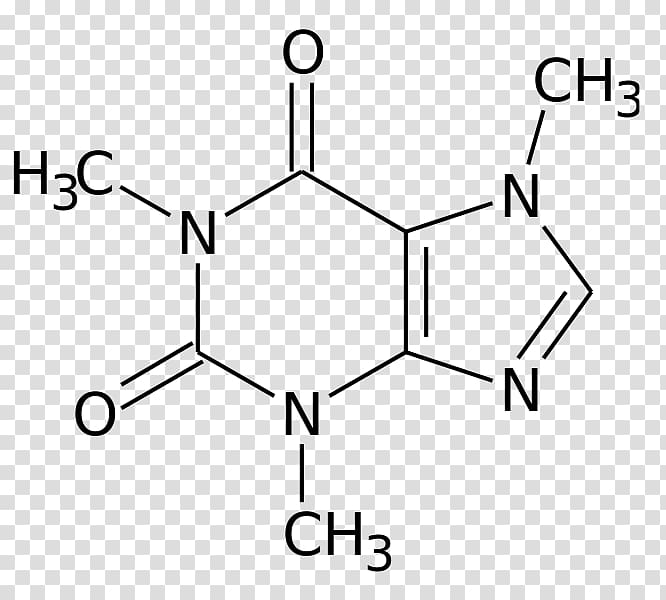
For example, additional methyl groups can help a molecule to cross the blood-brain barrier and enter our brain - this barrier protects our brain from foreign molecules traveling in the blood that can be harmful. It may seem simple, but a methyl group is an integral part of chemistry, biology, and biochemistry. At the molecular level, a methyl group is a carbon with three hydrogens attached. It seemed to make sense that as the caffeine I drank was metabolized by removing the methyl group, caffeine would convert to theobromine (the main compound of chocolate) (Figure 1).

When my organic chemistry professor told me that the main molecular component of chocolate, theobromine, differs from caffeine only by the absence of one methyl group I was delighted: I could skip an entire step in caffeine metabolism, avoid the bitter taste of coffee, and increase my chocolate consumption. These metabolites are then processed further before finally being excreted.Photo credit: Lisa Townley (left) Pyogenes Gruffer (right), Flickr. First, it is metabolized in the liver by cytochrome P450 enzymes into 3 metabolites, each with further physiological effects. The body quickly recognizes that caffeine is a foreign chemical and begins the process of removing it. Okay, we’ll finally get to the part all of you have been waiting for how much caffeine will kill you? Caffeine enters the bloodstream quickly and is an antagonist of the adenosine receptor in the brain, causing constriction of blood vessels and increasing blood pressure by blocking the effects of adenosine (vasodilation) 2.
This is the reason why espresso machines and devices like the Aeropress exist! They utilize high pressures so that caffeine and aromatic chemicals can be extracted from even the lightest of roasted beans. If the coffee bean roast is too light, these methods aren’t capable of extracting enough aroma and chemicals for a decent cup of coffee. Well, most regular coffee drinkers make their coffee using soluble forms or through a drip system systems that produce coffee under low pressures. You might be wondering why dark roasts even exist since they have less caffeine and aroma than lighter roasts. This is the reason why absurdly dark roasts lose much of their aroma. However, the majority of other chemicals present in coffee-that give the aroma and taste characteristic of the bean-are broken down much quicker. This means that very dark beans tend to have a lower caffeine content compared to lighter roasts. However, roasting the beans results in some caffeine decomposition. Caffeine: The Main Molecule Chemical StabilityĪs mentioned earlier, caffeine being stable means it is difficult to break down.


 0 kommentar(er)
0 kommentar(er)
